area in which an ESDS can be handled with acceptable risk of damage as a result of electrostatic discharge or fields
4 Electrostatic hazards
4.1 General
Four different hazards of static electricity are generally recognized: ESD damaging ordisrupting electrical equipment, contamination caused by ESA, igniti on of flammable substances and electrosta tic shock to peopl
4.2 ESD effects on equipment
Electrostatic discharges cancause losses of the functions of instrumentation during patient care increasing the risks to human safety. Insufficient electrostatic control may also cause unnecessary repair costs of medical equipment, as well as corruption of data affecting the quality and reliability of medical operation.
Electrical installation requirements for medical equipment and locations are provided in the electrical safety rules specified in IEC 60364(all parts)[3]. It is essential to recognize that electrical safety does not necessarily provide precautions for prevention of the risks of static electricity and electrostatic discharge (ESD).Local safety regulations shall be taken into account.
ESD immunity testing does not cover all the real discharge scenarios,such as those where metal partshaving different electric potentials are touched together. A current limiting resistor used in the ESD testing specified in IEC 61000-4-2 [4] does not necessarily exist in such situations, resulting inhigher discharge power in equipment under real stress. Charge accumulation in a mobile metal object can also result in high energies in uncontrolled environments. Especially in low humidity, discharge energies can exceed the stress levels used in IEC 61000-4-2 [4].
A completely integrated system in medical care is not necessarily tested against transients caused by ESD, although individual parts of the system have passed EMC qualification. Therefore, it does not always take into account all the realistic coupling and failure scenarios of the whole system.
Discharges from isolated conductors or from a human body can beprevented with grounding. Conductive parts of patient beds, intravenous stands, trolleys, delivery carts, over-bed tables, chairs, and other mobile metal objects are not normally connected to the protective earth. Therefore, grounding all conductive partsof every item through the flooring or with direct electrical connection to a functional ground becomes essential for static control.
The probability of ESD can efficiently be reduced by optimization of humidity levels, bipolar ionization,adequate material selection, personnel grounding, and grounding of mobile metal objects. In general, the prevention of static charge generation and ESD is preferable compared to enhancing medical equipment EMC immunity.
If a functional ground is used for electrostatic control purposes, it should be electrically bonded to protective earth where possible so as to avoid potential differences between the two systems.
4.3 Contamination caused by ESA
Electrostatically charged surfaces attract airborne particles. Increased depositionof microorganisms onto charged surfaces, including the airways, human skin, and open wounds, can contribute to the incidence of hospital infections. Electrostatic sources of contamination and nosocomial infection can be healthcare personnel, patients, or the environment. All objects that come into contact with patients can be considered as potentially contaminated.
Cleaning, disinfection and sterilizing can prevent transmission of infective agents. However, because of the human factor, complete certainty in cleanliness cannot be achieved without adequate control of the environment. Avoidance of electrostatic attraction (ESA) decreases airborne microbe contamination and improves overall cleanliness in healthcare. The reduction in charge carried by airborne submicron contaminants will additionally reduce the deposition of such contaminants in the airways, thereby reducing the load placed on the body’s immune system. Charge accumulation and high surface charge densities can be reduced to tolerablelevels by grounding of personnel and other conductors, and by correct selection of materials.
4.4 Ignition of flammable substances
The use of flammable substances in healthcare facilities has decreased, but the risk of fires and explosions can still occur especially in laboratories, intensive care units and operating rooms. For example, using alcohol based sterilizing substances has caused fires due to electrostatic discharge. ESD can be an ignition source in hyperbaric oxygen facilities and other locations where the oxygen concentration exceeds 23,5 % by volume.
The risk of incendiary ESD can be reduced to tolerable levels by grounding of personnel and other conductors, and by correct selection of materials.
4.5 Electrostatic shock to people
The incidence of unpleasant electrostatic shocks to peoplehas increased due to the increased use of highly insulating materials such as plastics. Anelectrostatic discharge occurs when a human body approaches close enough to an object with different electric potential to exceed the electric breakdown field strength. ESD energy can be high enough to cause painful sensations to patients and healthcare personnel, resulting in involuntary movements, which can lead to accidents.
The risk of electrostatic shock can be reduced to tolerable levels by grounding of personnel and other conductors, and by correct selection of materials
5 Electrostatic control requirements
5.1 General
Electrostatic control requirements in healthcaredepend on the medical procedures, locations and activities such as service and maintenance of medical equipment.
5.2 Medical procedures
To ensure safetyof patients from electrostatic hazards, protective measures shall be applied during medical examination and treatment.
Medical procedures can require specific electrostatic control actions that are dependent on the particular requirements of instrumentation, electric equipment or cleanliness. When protective measures have not otherwise been specified, the requirements in 5.3 to 5.7 shall be applied. Consideration shall also be given to applying the recommendations in 5.3 to 5.7a nd Annex B.
5.3 Medical locations
5.3.1 Classification by groups
Locations that are intended for purposes of diagnosis, treatment, monitoring and care of patients shall be classified in the following groups, as defined in IEC 60364-7-710: unclassified, G0, G1 and G2.
Selection of materials to reduce residual charge levels is recommended in all locations. Grounding of personnel and other conductors is recommended in G0 and G1 locations, and is required in G2 locations. Electrostatic control methods for each location are summarised in Table 1.
Table 1 - Summary of electrostatic control methods for specified locations
[...]
5.6 Technical requirements
5.6.1 Electrical safety
This documentincludes technical requirements forelectrostatic control. These requirements shall notreplace or supersede any requirements for personnel safety.
5.6.2 Material classification
5.6.2.1 General requirements for material classification
Materials for electrostatic control shall: limit the generation of electrostatic charge; quickly dissipate electrostatic charge; suppress or attenuate electrostatic field or electrostatic potential associated with residual electrostatic charge. To a certain extent, these functions are related. For example, a material thatis able to dissipate charge faster than it is generated will appear to be one that limits the generation of charge. However, in some cases, the functions can be independent. Some materials that do not dissipate charge quickly, can show limited accumulation of charge or low measured surface potential
...
Annex A (normative)Test methods for low charging textiles
A.1 Test methods for clothing and upholstery
The test methods specified in IECTS 61340-4-2 shall be used for triboelectric charging measurements relating to people, clothing and upholstery. With minimum modification, the test methods shall also be used for triboelectric charging measurements relating to people, bedding and surgical drapes.
In all cases the measured body voltage shall be ≤ 2 000 V. The resistance to ground of the person or mannequin shall be greater than 1013Ω during all measurements. The additional measurements and calculations described in IECTS 61340-4-2 are not essential for the purposes of this document.
Triboelectric charging tests require the product under test to be contacted by other materials, which shall either be representative of the materials most likely to comeinto contact with the product in use, or reference materials that are known to generate high levels of charge when rubbed against other materials. In the latter case, at least two reference materials shall be used: one electropositive and one electronegative. Examples of suitable material include (going from electropositive toelectronegative) polyamide, wool, leather, polyester, polyvinylchloride and polytetrafluoroethylene.
Clothing shall be tested according to the method specified in IECTS 61340-4-2:2013, Annex A, Annex B or Annex C. The clothing used shall be the combinations of clothing under test, for examplea surgical apron and a surgical gown, or combinations of the clothing under test and reference clothing or materials.Upholstery shall be tested according to the method specified in IECTS 61340-4-2:2013, Annex B.
The upholstery under test shall be installed on samples of the furniture to be used, or on representative reference furniture. The clothing used shall be representative of the clothing most likely to contact the upholstery in use or reference clothing.
A.2 Test methods for bedding, curtains, and surgical drapes Bedding, curtains and surgical drapes shall be tested according to the method specified in IECTS 61340-4-2 by replacing the seat with a bed or examination/operating table. The clothing used shall be representative of the clothing most likely to contact the product under test when in useor reference clothing. Alternatively, tests shall be made according to the following methods based on IECTS 61340-4-2:2013,Annex A and Annex B, as illustrated in Figures A.1 to A.3.
In the example shown in Figure A.1, a person wearing clothing that is representative of clothing likely to be worn within the intended application, or reference clothing, lies on a bed or examination/operating table. A thin sheet of insulating material, for examplepolyethylene film, shallbe placed beneath the person so that the resistance from the person to ground is greater than 1013Ω. The product under test is placed over the person, who is then momentarily grounded. The product under test is then quickly removed and the body voltage of the person on the bed is recorded.
In the example shown in Figure A.2, the products under test can be the bedding or surgical drape removed, or the bedding or cover on the bed or examination/operating table, or both. In this example, the person conducting the test stands without footwear on a metal plate connected to the electrostatic voltmeter. The resistance from the metal plate to ground shall be greater than 1013Ω. After the item is removed, it is held close to the body and shall be prevented from touching anything else. The body voltage of the person is recorded.In the example shown in Figure A.3, the two procedures are combined.
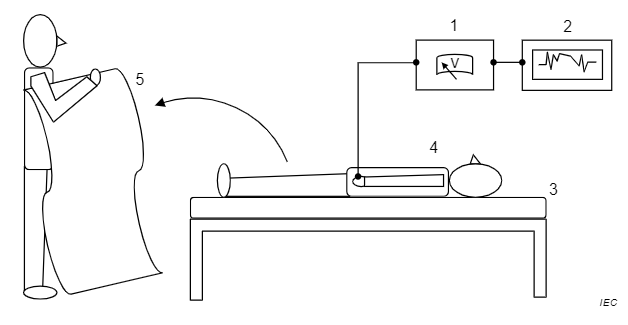
Key
1 electrostatic voltmeter
2 recording device
3 bed or examination/operating table
4 person wearing reference clothing, resistance to ground > 1013Ω
5 bedding or surgical drape under test
Figure A.1 - Example of test equipment set up for measuring body voltage when removing item of bedding or surgical drape from person wearing reference clothing
[...]
segue in allegato
Fonti:
CEI EN IEC 61340-6-1
Elettrostatica Parte 6-1: Controllo elettrostatico in ambito sanitario - Prescrizioni generali per infrastrutture
Certifico Srl - IT | 2019
©Copia autorizzata Abbonati



