// Documenti disponibili n: 46.588
// Documenti scaricati n: 36.655.248
In aggiornamento
Diagram of the procedures for the placing on the market of machinery and partly completed machinery.
The following diagram summarises the procedures set out in Article 12 and 13:
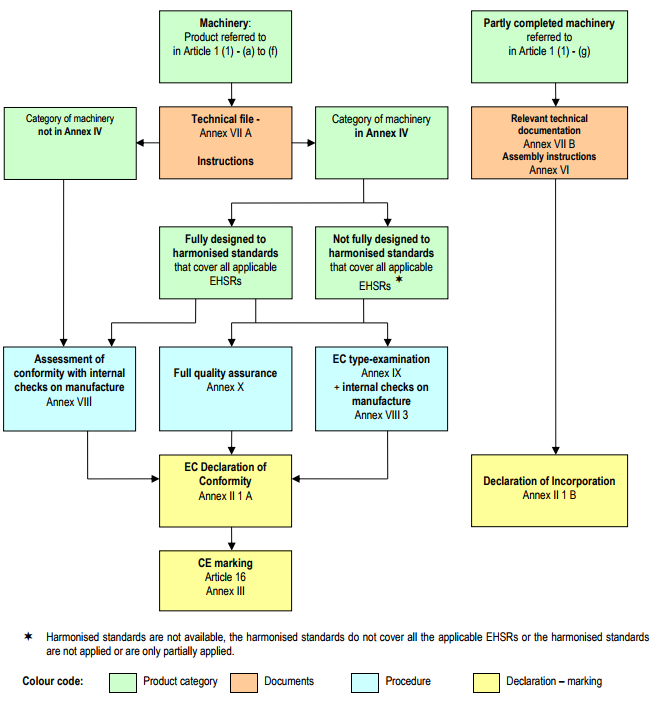
1. The manufacturer or his authorised representative shall, in order to certify the conformity of machinery with the provisions of this Directive, apply one of the procedures for assessment of conformity described in paragraphs 2, 3 and 4.
2. Where the machinery is not referred to in Annex IV, the manufacturer or his authorised representative shall apply the procedure for assessment of conformity with internal checks on the manufacture of machinery provided for in Annex VIII.
3. Where the machinery is referred to in Annex IV and manufactured in accordance with the harmonised standards referred to in Article 7(2), and provided that those standards cover all of the relevant essential health and safety requirements, the manufacturer or his authorised representative shall apply one of the following procedures:
(a) the procedure for assessment of conformity with internal checks on the manufacture of machinery, provided for in Annex VIII;
(b) the EC type-examination procedure provided for in Annex IX, plus the internal checks on the manufacture of machinery provided for in Annex VIII, point 3;
(c) the full quality assurance procedure provided for in Annex X.
4. Where the machinery is referred to in Annex IV and has not been manufactured in accordance with the harmonised standards referred to in Article 7(2), or only partly in accordance with such standards, or if the harmonised standards do not cover all the relevant essential health and safety requirements or if no harmonised standards exist for the machinery in question, the manufacturer or his authorised representative shall apply one of the following procedures:
(a) the EC type-examination procedure provided for in Annex IX, plus the internal checks on the manufacture of machinery provided for in Annex VIII, point 3;
(b) the full quality assurance procedure provided for in Annex X.
1. The manufacturer of partly completed machinery or his authorised representative shall, before placing it on the market, ensure that:
(a) the relevant technical documentation described in Annex VII, part B is prepared;
(b) assembly instructions described in Annex VI are prepared;
(c) a declaration of incorporation described in Annex II, part 1, Section B has been drawn up.
2. The assembly instructions and the declaration of incorporation shall accompany the partly completed machinery until it is incorporated into the final machinery and shall then form part of the technical file for that machinery.
Collegati
Guida Direttiva macchine 2006/42/CE - Ed. 2017 EN
ID 1606 | Rev. 1.0 2019
Quando la valutazione dei rischi evidenzia che una macchina o un processo comporta un rischio di lesioni, il pericolo d...

Check list Non Conformità di marchi, documenti, caratteristiche
Una breve guida per l'identificazione di Gru mobili non conformi
Marcatura delle macchine, documenti, carat...

ID 19804 | Ed. 3.0 del 09.11.2024 / formato pdf navigabile con link / epub
Disponibile in formato pdf /epub navigabile e st...
Testata editoriale iscritta al n. 22/2024 del registro periodici della cancelleria del Tribunale di Perugia in data 19.11.2024