// Documenti disponibili n: 46.591
// Documenti scaricati n: 36.657.803

STEP 1 - Identify the directive(s) and harmonized standards applicable to the product
There are more than 20 directives setting out the product categories requiring CE marking. The essential requirements that products have to fulfil (e.g. safety) are harmonised at EU level and are set out in general terms in these directives. Harmonised European standards are issued with reference to the applied directives and express in detailed technical terms the essential requirements.
STEP 2 - Verify the product-specific requirements
It is up to you to ensure that your product complies with the essential requirements of the relevant EU legislation.Full compliance of a product to the harmonised standards gives a product the “presumption of conformity” with the relevant essential requirements. The use of harmonized standards remains voluntary. You may decide to choose other ways to fulfil these essential requirements.
STEP 3 - Identify whether an independent conformity assessment is required from a Notified body
Each directive covering your product specifies whether an authorised third party (Notified Body) must be involved in theconformity assessment procedure necessary for CE marking. This is not obligatory for all products, so it is important to check whether the involvement of a Notified Body is indeed required. These Bodies are authorised by national authorities and officially “notified” to the Commission and listed in the NANDO (New Approach Notified and Designated Organisations) database.
STEP 4 - Test the product and check its conformity
Testing the product and checking its conformity to the EU legislation (Conformity Assessment Procedure) is the responsibility of the manufacturer. One part of the procedure is, as a general rule, a risk assessment. By applying the relevant harmonised European standards, you will be.
STEP 5 – Draw up and keep available the required technical documentation
The manufacturer has to establish the technical documentation required by the directive(s) for the assessmentof the product’s conformity to the relevant requirements, and for the risk assessment. Together with theEC declaration of conformity, the technical documenta tion must be presented on request to the appropriate national authorities.
STEP 6 – Affi xation of the CE marking to your product and EC Declaration of Conformity
The CE marking must be affixed by the manufacturer, or by his authorized representative within the EEA or Turkey. It must be affixed according to its legal format visibly, legibly and indelibly to the product or its data plate. If a Notified Body was involved in the production control phase, its identification number must also be displayed. It is the manufacturer’s responsibility to draw up and sign an “EC declaration of conformity” proving that the product meets the requirements.
That’s it! Your CE-marked product is ready for the market.
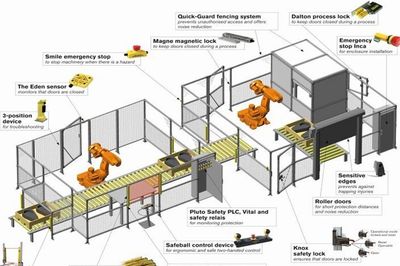
Se non è possibile ottenere una riduzione dei rischi soltanto tramite la progettazione, si rende necessario l'impiego di parti di comando ...

Part 1: Mills, crushers, mixers, separators, screeners
Compendium for Practical Use
The ISSA (International Social Security A...

ID 3370 | 28.12.2016
Norma EN ISO 14119:2013. Caso studio
Il documento è nato da una collaborazione tra Inail (Laboratorio macchine ed at...
Testata editoriale iscritta al n. 22/2024 del registro periodici della cancelleria del Tribunale di Perugia in data 19.11.2024