// Documenti disponibili n: 46.588
// Documenti scaricati n: 36.655.283
This Vademecum compiles some key documents from the Commission services on European standardisation policy and related practice.
It provides guidance without having legal status.
The main aim of the Vademecum is to serve as a guide for Commission officials. It will enable them to use, as appropriate, European standardisation as a tool for the implementation of the European policies and legislation for which they are responsible.
The Vademecum also seeks to guide the Member States. It will help them to understand the Commission's European standardisation policy and related practice and to know the mechanisms of referring to European standards in European policies/legislation.
Finally, this guide could also be used by stakeholders in standardisation (such as standardisers, enterprises and non-governmental organisations) to gain a clear picture of the policies and processes of the Commission in this field.
The publication of the Vademecum makes the policy of the European Commission on European standardisation more transparent and accessible to the outside world.
Suggestions for improvement are always welcome on
Table of contents
Introduction
Foreword pdf - 14 KB [14 KB]
General framework
Standardisation and the Directive 98/34/EC
Historical background pdf - 58 KB [58 KB]
Basic principles (to be added later)
General Guidelines for the Cooperation between CEN, CENELEC and ETSI and the European Commission and EFTA - Explanatory note (to be added later)
European Standardisation in support of European Policies
Standardisation Setting and Governance pdf - 64 KB [64 KB]
Methods for Reference to Standards in European Legislation pdf - 232 KB [232 KB]
Models of articles on standardisation issues to include a draft Directive pdf - 19 KB [19 KB]
Role, Elaboration and Follow-up of Mandates
Role and elaboration of Mandates pdf - 85 KB [85 KB]
Follow-up of Mandates pdf - 420 KB [420 KB]
Publication of References of Standards in the Official Journal
Guide to the implementation of Directives based on New Approach and Global Approach (blue guide)
Guidelines for publication pdf - 251 KB [251 KB]
Procedure for Formal Objections against Standards pdf - 48 KB [48 KB]
Co-financing by EU/EFTA of European Standardisation
Descriptive document on financing methods and referencing to the foreseen Framework Partnership Agreement pdf - 36 KB [36 KB]
European Standardisation in the International Context
Strategy for the international promotion of standards pdf - 27 KB [27 KB]
TBT code and standards pdf - 21 KB [21 KB]
European Policy Principles on International Standardisation [SEC(2001) 1296 of 26 July 2001]
The external dimension of the Internal Market
Overview pdf - 35 KB [35 KB]
Implementing Policy Principles for External Trade in the fields of Standards & Conformity Assessment: A Toolbox of instruments pdf - 108 KB [108 KB]
Agreements on Conformity Assessment and Acceptance of Industrial Products (to be added later)
Technical Assistance and Standardisation
General description (to be added later)
European Community TBT related technical assistance to developing countries - EC submission to the TBT committee pdf - 27 KB [27 KB]
Technical Assistance and capacity building in the field of Technical Barriers to Trade pdf - 36 KB [36 KB]
Annexes
Regulation (EU) No 1025/2012
General Guidelines for the Cooperation between CEN, CENELEC and ETSI and the European Commission and EFTA pdf
Directives and Regulations under New Legislative Framework (NLF)
References of harmonised standards and of other European standards published in the OJEU
Standardisation related websites
http://ec.europa.eu/enterprise/policies/european-standards/documents/vademecum/index_en.htm

Report 41 del 14/10/2016 N.25 A12/1289/16 Regno Unito
Approfondimento tecnico: Thermos
Il prodotto, di marca Thermate, Mod. 0-57024, è stato sotto...

Report 07 del 19/02/2016
N.13 A12/0166/16 Italia
Approfondimento tecnico: Pigmento per tatuaggio
Il prodotto “pigmento per tatuaggio”, marca Etern...
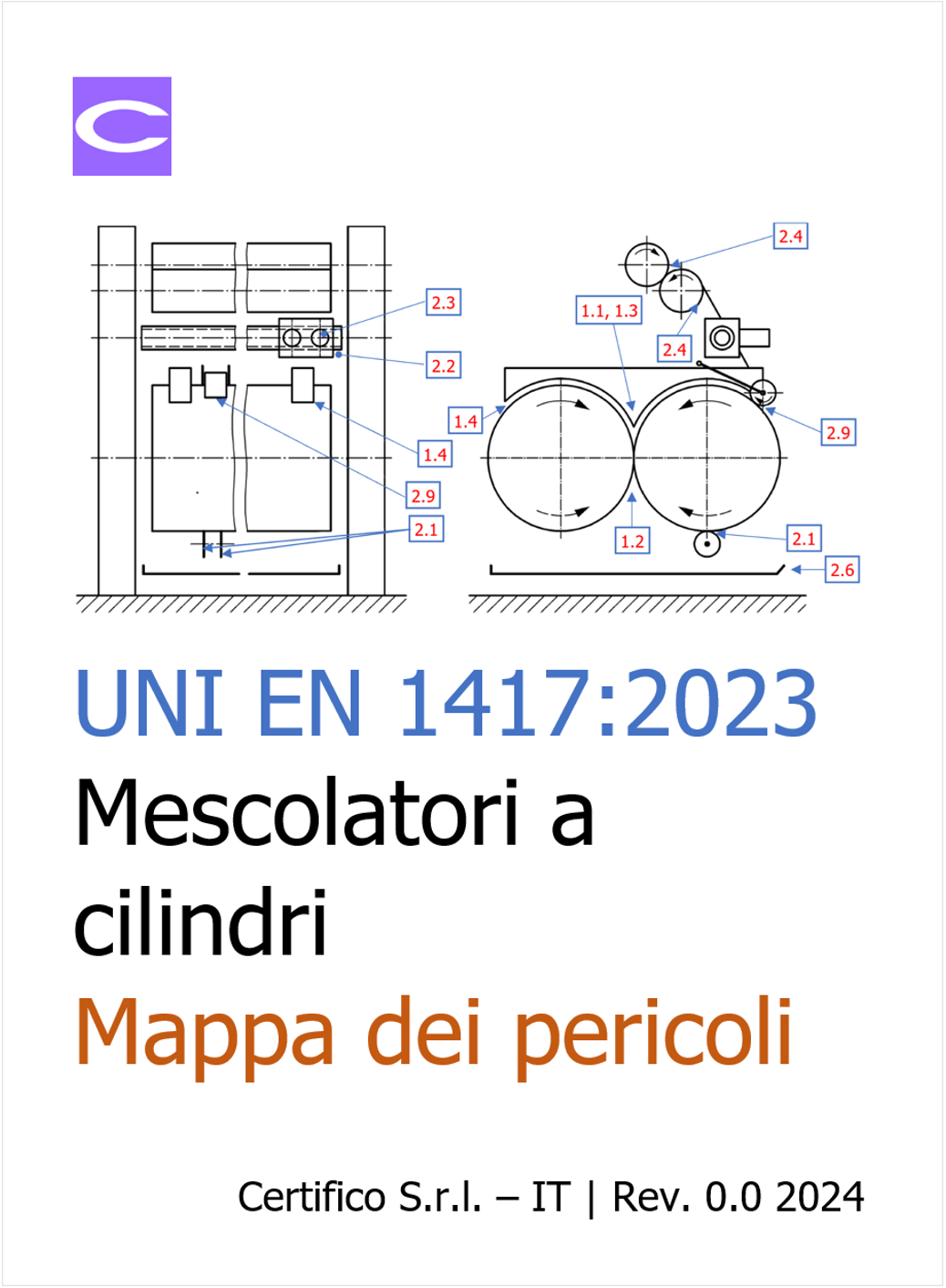
ID 21215 | 01.02.2024 / Documento di approfondimento allegato
Documento di approfondimento sui pericoli dei mescolatori a cilindri per mater...
Testata editoriale iscritta al n. 22/2024 del registro periodici della cancelleria del Tribunale di Perugia in data 19.11.2024