Restrictions Roadmap under the Chemicals Strategy for Sustainability
| ID 16498 | | Visite: 1329 | Documenti Chemicals UE | Permalink: https://www.certifico.com/id/16498 |
Restrictions Roadmap under the Chemicals Strategy for Sustainability
ID 16498 | 27.04.2022 / In allegato documento completo
Brussels, 25.4.2022 - SWD(2022) 128 final
On 14 October 2020, the European Commission published its chemicals strategy for sustainability towards a toxic-free environment (the ‘strategy’) as part of the European Green Deal. The strategy highlights that chemicals are fundamental for society and a robust framework is needed to make Union legislation stronger and more coherent. It presents several actions to bring about a toxic-free environment and to protect people and the environment from hazardous chemicals.
In particular, the Commission is considering extending the ‘generic approach to risk management’, i.e. restricting certain substances in products for certain users while allowing limited exemptions under conditions clearly defined in law.
Until the proposed changes have been assessed and introduced in Regulation (EC) No 1907/2006 (REACH Regulation), the strategy aims to ‘prioritise carcinogenic, mutagenic and reprotoxic substances (CMRs), endocrine disruptors, persistent, bioaccumulative and toxic (PBT) and very persistent and very bioaccumulative (vPvB) substances, immunotoxicants, neurotoxicants, substances toxic to specific organs and respiratory sensitisers substances for (group) restrictions’ for all uses. To facilitate this action, the Commission has prepared a roadmap to prioritise these substances for (group) restrictions under REACH (the ‘Restrictions Roadmap’).
In its Conclusion on the strategy (in para. 21), the Council stated that it:
- ‘supports the prioritisation of restrictions for the most harmful chemicals to be covered by the generic approach for all uses and through grouping as an interim solution until the extension of the generic approach to risk management is fully implemented
- stresses that the Member States should also be able to initiate restrictions based on this approach’.
A first exchange on a draft Restrictions Roadmap took place at the Risk Management and Evaluation (RIME+) meeting on 22 April 2021 and the Restrictions Task Force meeting on 29 April 2021. Revised drafts were subsequently discussed at the CARACAL (Competent Authorities for REACH and CLP) meetings of 28 and 29 June 2021 and on 17 and 18 November 2021.
This document is a staff working document prepared by the Commission services and does not necessarily represent the views of the European Commission. It is in no way legally binding.
Objectives of the Restrictions Roadmap
The Restriction Roadmap has three main objectives:
1. Ensure that the commitments under the strategy can be fulfilled in a transparent and timely manner. The Rolling List included in the Annex (see below) sets out the restrictions that have been planned and prepared, and those that have progressed, in particular for the most harmful substances (i.e. those that meet the criteria for CMRs, PBTs, vPvBs, endocrine disruptors (ED), immunotoxicants, neurotoxicants, respiratory sensitisers and STOT substances (Specific target organ toxicity). It will be the cornerstone for the multiannual planning under Article 68 of REACH on introducing new and amending current restrictions and Article 69 of REACH on preparing proposals for the period up to 2025-2027, until the new rules on the generic approach are put in place.
2. Provide an overview, through its Rolling List, of we are using the available authority resources. The Rolling List contains (groups of) substances which are being considered for a risk management measure or for which an entry in the Registry of Intentions (RoI) has been submitted.
3. Provide transparency to stakeholders on the restriction work by authorities and allows companies to anticipate (potential) upcoming restrictions, e.g. by already beginning substitution activities.
Those restrictions aim to maximise the reduction of unacceptable chemical risks with all available resources, by means of broader restrictions, through both grouping of substances, and addressing a wider range of uses (industrial, professional, consumer uses and uses in articles). This should lead to better cooperation and shared work to ensure that authority resources are helping to fulfil the overall aim of the Roadmap in an optimal way.
In this process, two important conditions are to be underlined:
1. The Rolling List will be regularly reviewed. Further investigations may lead to changes in the anticipated regulatory risk management action. Therefore, it is ‘rolling’ in nature and substances covered by the Restrictions Roadmap may finally not be restricted in practice and may be taken off the list while other substances may be added.
2. The Roadmap, including the Rolling List, will be established without affecting Member State prerogatives under REACH. Thus, the Roadmap does not affect the Member States’ right to propose new restrictions, including those for substances not (yet) included in the Roadmap.
The Roadmap should therefore provide for a balance between the need for flexibility on when and how to act while securing the necessary commitment to ensure progress on restricting the most harmful (groups of) substances as set out in the strategy. The roadmap will further guide the prioritisation of substances for which safe and sustainable alternatives should be developed according to the criteria on Safe and Sustainable by Design (SSbD) announced in the chemical strategy for sustainability and to be published by the Commission in 2022.
Implementing the Roadmap will require the joint commitment and collaborative efforts of Member States, the Commission and ECHA (European Chemicals Agency (ECHA). Achieving the Roadmap’s objectives requires ECHA and the Member States to have adequate resources to work on further RMO (Risk Management Option) analysis (if needed), hazard confirmation (if needed) and restriction work.
The Rolling List will be discussed periodically at CARACAL and in principle be updated once per year.
[...]
1. Context
2. Objectives of the Restrictions Roadmap
3. Identifying (groups of) substances for the Restrictions Roadmap Rolling List
a. Sources of information for Commission and Member States to begin restrictions.
b. Assessment of risk from the use in articles of substances on the Authorisation List under Article 69(2) of REACH
4. The Rolling List of (groups of) substance(s) for restriction
Annex I - Rolling List of (groups of) substances for restriction
Annex II - Overview of Article 69(2) assessments
_______
Annex I - Rolling List of (groups of) substances for restriction
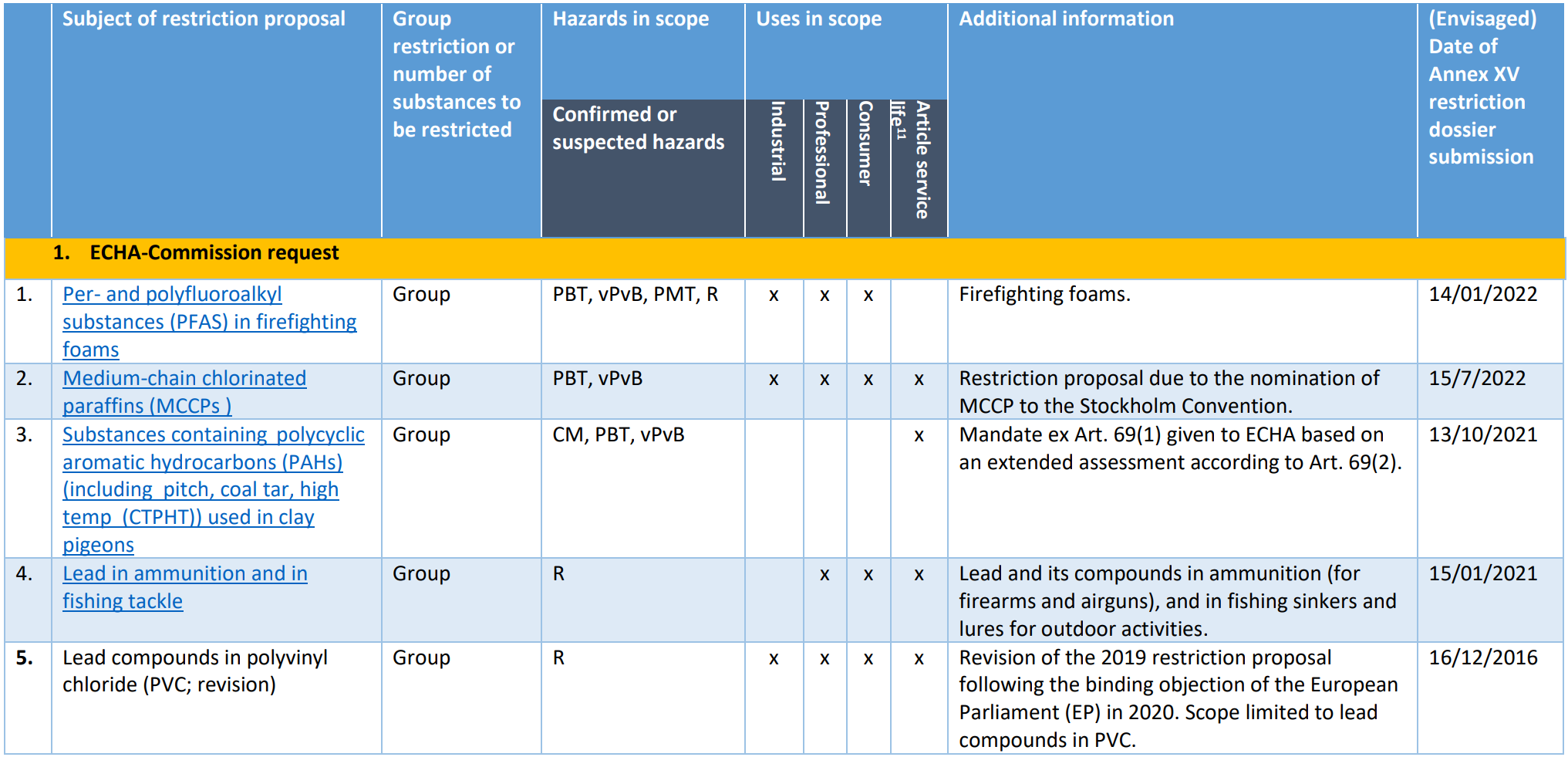
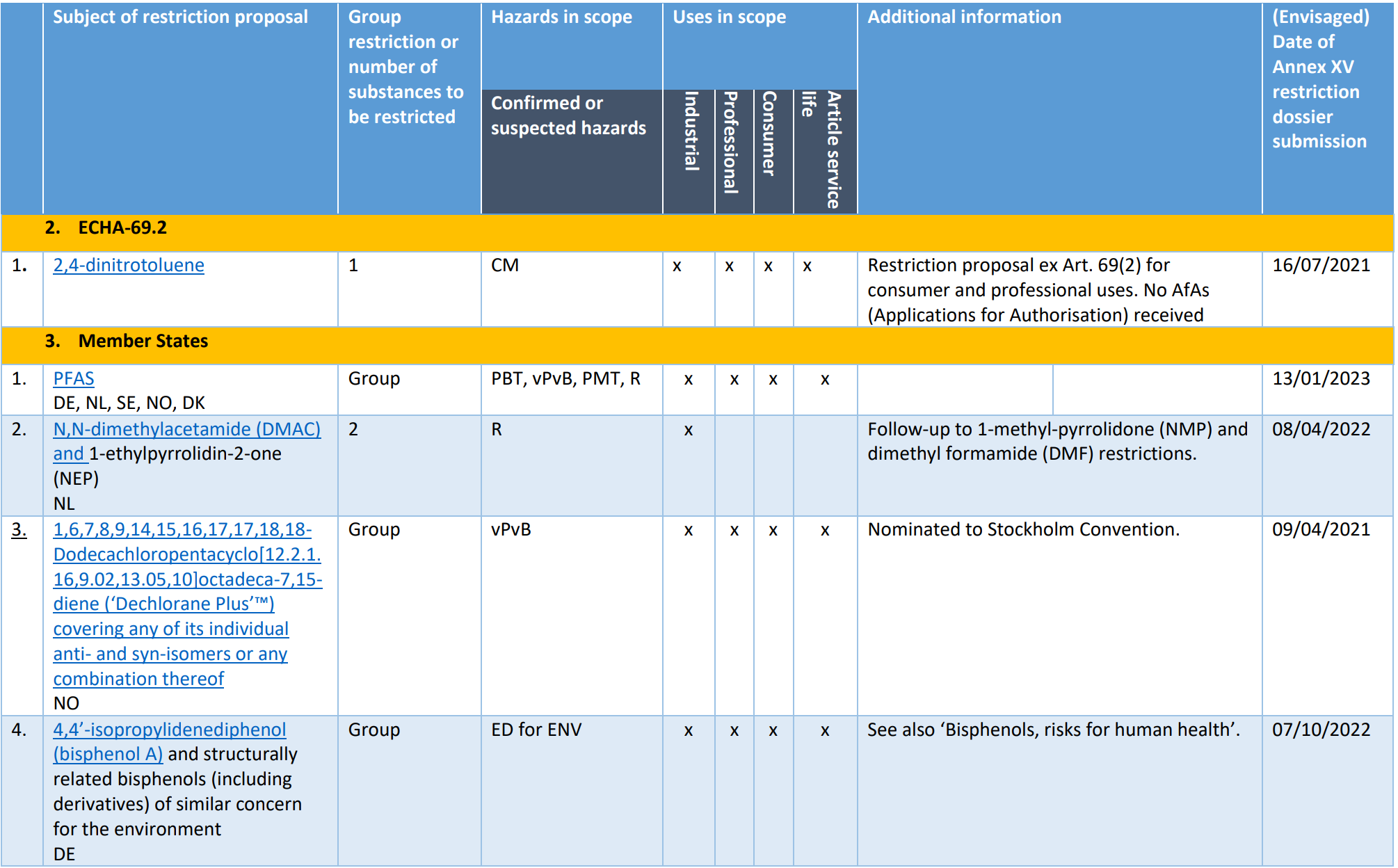
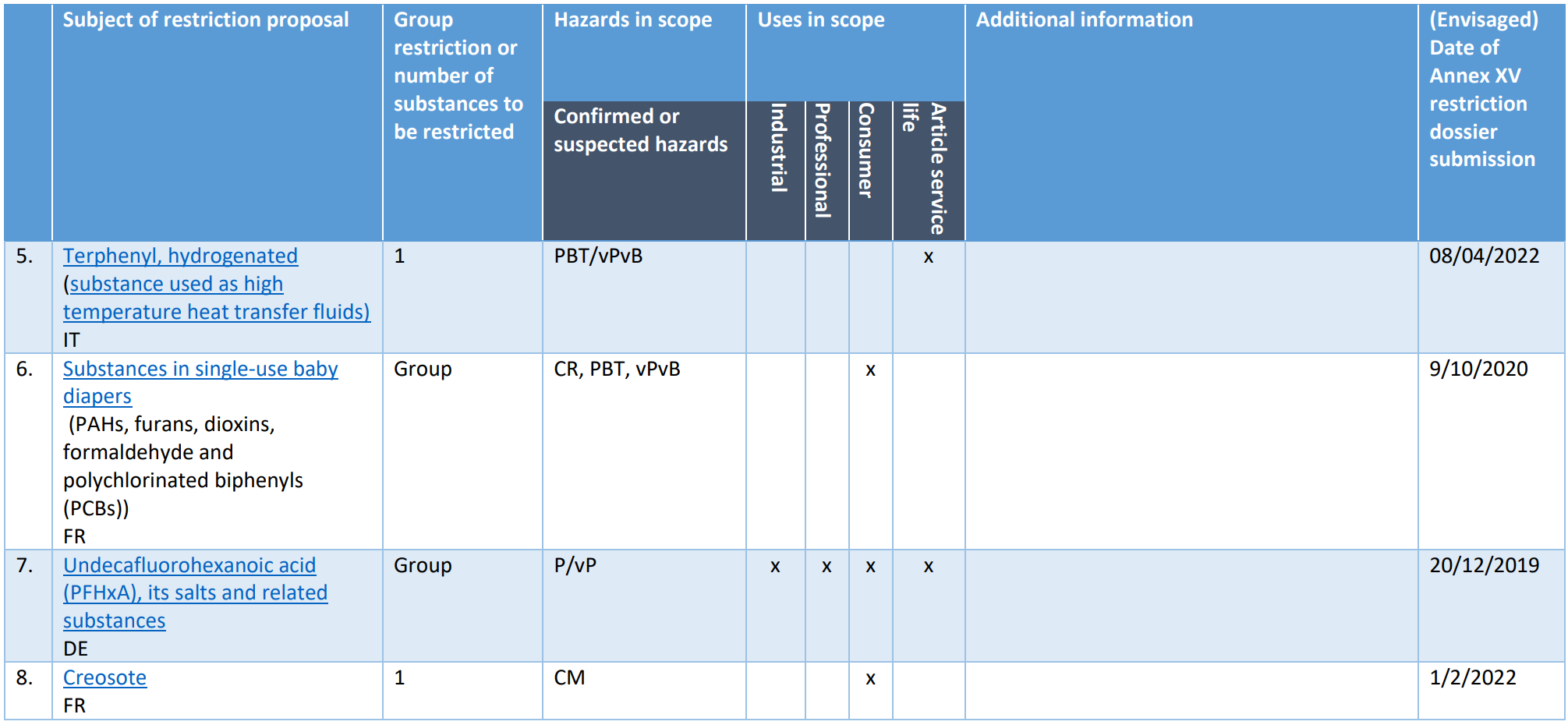
Pool 0: Restrictions already on the Registry of Intention (RoI), mandate provided to ECHA or restriction dossier recently submitted
[...] Segue in allegato
Fonte: CE
Collegati
| Descrizione | Livello | Dimensione | Downloads | |
|---|---|---|---|---|
| Restrictions Roadmap under the Chemicals Strategy for Sustainability.PDF CE - 25.04.2022 |
1230 kB | 9 |
Tags: Chemicals Reach Abbonati Chemicals